Abstract
Introduction
Consolidating high-dose chemotherapy with melphalan followed by autologous stem cell transplantation (ASCT) is believed to be superior to conventional chemotherapy alone in fit multiple myeloma patients ≥65 years. However, contemporary high quality data on the overall efficacy and safety of ASCT in this elderly age group are missing, and the conditioning regimen including the optimal melphalan dose is a matter of debate. Our present study aims at addressing the issue of the melphalan dosing prior to ASCT in patients ≥ 65 years of age.
Methods
This is an observational, multi-center, non-interventional research project based on data of the prospective Swiss Blood Stem Cell Transplantation Registry. Included are all Swiss patients ≥65 years with multiple myeloma who received high-dose chemotherapy followed by ASCT. Patients receiving melphalan at a dose of 200mg/sqm, and patients receiving melphalan at a dose of < 200mg/sqm were analyzed separately. For the 53 patients receiving double ASCT, only the first ASCT was considered, and survival was censored at the time of second ASCT. The main outcomes analyzed in the study were treatment-related mortality prior to day 100 (TRM), progression free survival (PFS), and overall survival (OS) estimates at two and five years.
Results
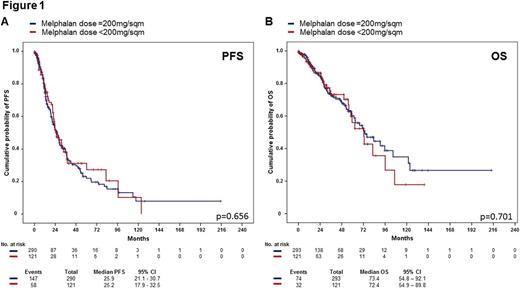
Between 2000 and 2016, 414 patients with multiple myeloma aged 65 years and older received high-dose chemotherapy with melphalan and ASCT. Of those, 165 patients (40%) were female. The median age at ASCT was 67 years (range 65-77 years), and the median observation time of the surviving patients was 19 (range 0-215) months. Monoclonal component subtype was IgG (n=247, 60%), IgA (n=90, 22%), light chain (n=65, 16%), and others (n=12, 2%), respectively. Risk stratification according to the International Staging System (ISS) defined stage I in 136 (38.1%) patients, stage II in 144 (40.3%) patients, and stage III in 77 (21.6%) patients. Cytogenetic information was available for 212 (51.2%) patients, with 44 (20.8%) patients presenting high-risk features defined by the presence of del(17p), t(4;14), t(14;16), or combinations of these. Remission status prior to transplant was complete response (n=50, 12%), very good partial response (n=105, 25%), and partial response (n=229, 55%), respectively. Most patients had a Karnofsky Performance Status (KPS) of 90-100% prior to ASCT (n=318, 80.3%), while a KPS ≤ 80% was observed in 78 (19.7%) patients. Two hundred ninety-one patients (70%) received a melphalan dose of 200mg/sqm, and 123 patients (30%) received less than 200mg/sqm (median 140mg/msq, range 70 to 180mg/sqm). Patients who received melphalan doses <200mg/sqm were slightly older (67.8 years vs. 67.3 years, p=0.004), and had higher serum creatinine levels (80 µmol/l vs. 74 µmol/l, p<0.001), but did not differ with regards to ISS stage, cytogenetic risk group, remission status, and KPS prior to ASCT. At day 100, seven patients (1.7%) had died due to transplant-related complications, without differences between the two melphalan dose groups (p=0.429). Median PFS in the melphalan 200mg/sqm group was 25.9 months (21.1-30.7 months), and in patients receiving lower melphalan doses 25.2 months (17.9-32.5 months), respectively (p=0.656) (Figure1A). Estimated overall survival at 2 years and 5 years were 84.1% (79.0-89.2%), and 60.2% (51.4-69.0%) in the melphalan 200mg/sqm group, as compared to 85.1% (77.6-92.5%) and 56.0% (40.5-71.5%) in patients receiving lower melphalan doses (p=0.701) (Figure 1B). In a multivariate analysis adjusted for renal function, ISS stage, age at ASCT, and KPS, the melphalan dose was not associated with differences in the PFS or the OS.
Conclusions
The present study shows that high-dose melphalan and ASCT is feasible and safe in an elderly patient population with multiple myeloma. No significant differences in TRM, PFS and OS were observed between patients treated with melphalan 200mg/sqm, and patients treated with lower melphalan doses.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal